A PCR master mix is a convenient mixture of all essential reagents needed for a PCR reaction. Proper preparation of the master mix ensures consistent, reproducible, and accurate amplification. In this guide, we’ll share recipes, tips, and common mistakes to avoid when preparing PCR master mixes for your experiments.
What is a PCR Master Mix?
A PCR master mix contains:
DNA polymerase (e.g., Taq polymerase)
dNTPs (building blocks for DNA synthesis)
MgCl₂ (cofactor for polymerase)
PCR buffer (stabilizes pH and ionic strength)
Primers (forward and reverse)
Optional additives (enhancers or stabilizers)
Using a master mix reduces pipetting errors, minimizes variability between reactions, and saves time when running multiple PCRs. Read more
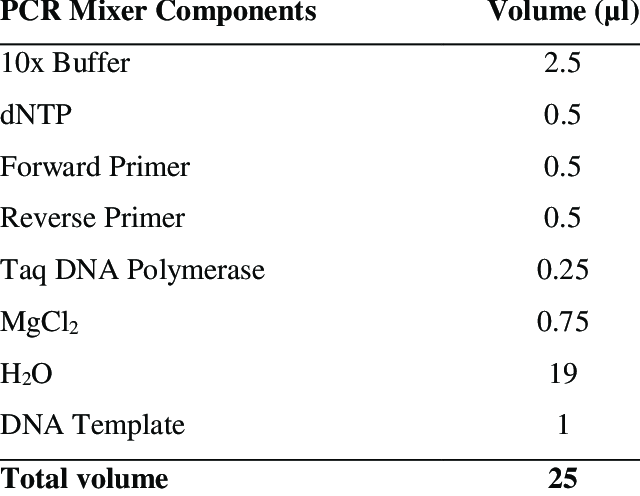
Basic PCR Master Mix Recipe (25 µL Reaction)
Component |
|
|
|
| 2.5 |
|
| 0.5 |
|
| 0.5 |
|
| 0.5 |
|
|
|
| Taq DNA polymerase (5 U/µL) |
|
|
|
|
|
|
|
| |
Tip: Always prepare a master mix for multiple reactions at once to reduce pipetting errors.
Tips for Preparing an Accurate PCR Master Mix
Keep components on ice to preserve enzyme activity.
Mix gently; avoid creating bubbles which can affect reaction efficiency.
Use nuclease-free water to prevent degradation of DNA or RNA templates.
Prepare slightly more volume than needed to account for pipetting loss.
Include controls: No Template Control (NTC) to check for contamination.
Troubleshooting PCR Master Mix Issues
Problem |
|
|
|
| | Double-check all components |
|
| | Too much template or Mg²⁺ |
|
|
| | Incorrect annealing temperature |
| | Optimize temperature and primer design |
|
| | Pipetting inconsistencies |
| | Use a master mix for all reactions |
|
Conclusion
Proper preparation of a PCR master mix is crucial for successful amplification and reproducibility. By following this guide and using standardized recipes, you can reduce errors, save time, and improve the reliability of your PCR and qRT-PCR experiments.

